We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
Innovating Science® - Mole Ratio of Reactants
Rating:
80 % of 100
$39.75
Chemical products can only be purchased by Corporations and Educational Institutions. Private Individuals are not eligible.
Chemical purchases under $50 requires an additional $10 handling fee.
Manufacturer ID - IS8003
Students will learn about acids and bases through a class discussion and then test a dilute acid and dilute base with neutral litmus paper to learn how the paper indicates something is acidic or basic. Once that skill is learned they can move on to test some common items that would be found around their house to determine if they are acids or bases. Optional: Extra litmus paper is included to test more items either from home or around the school building for a greater understanding of acids and bases. The activity contains enough materials for 6 groups of students.
Description
Using the method of continuous variation, two solutions are combined in various ratios. To select the ratio that produces the most product or consumes the most reactants, students must find an empirical method which is proportional to the amount of reaction that occurs. The reaction selected for this experiment is exothermic and the optimum ratio produces the greatest temperature change.
In this experiment the total numbers of moles of reactants are kept constant while varying each reactant. The measurements are made on each different ratio until the optimum ratio, the stoichiometric ratio in the equation, is made which consumes the greatest amount of reactants, produces the greatest amount of product and produces the greatest amount of heat.
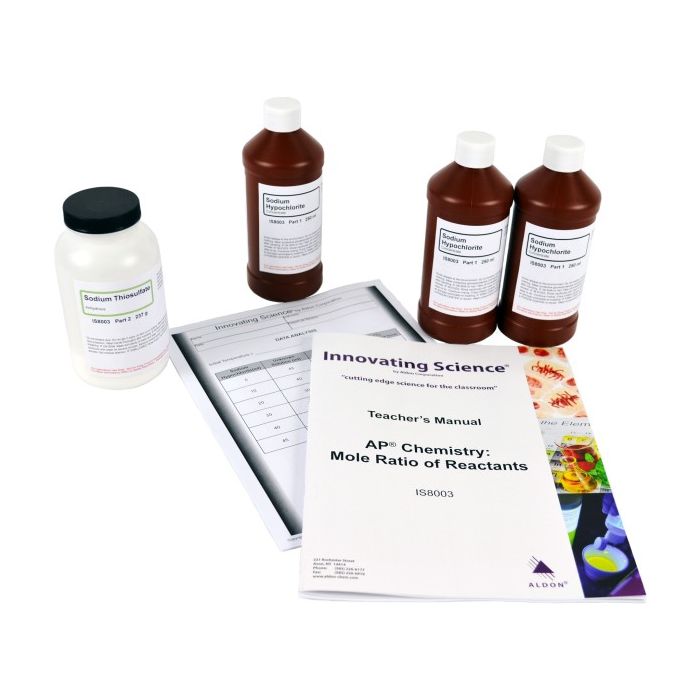
Kit Includes:
3 X 250mL Sodium Hypochlorite 13% Concentrate
1 X 237g Sodium Thiosulfate, anhydrous
DOT Info:
UN1791, Hypochlorite solutions, 8, III, Ltd Qty
In this experiment the total numbers of moles of reactants are kept constant while varying each reactant. The measurements are made on each different ratio until the optimum ratio, the stoichiometric ratio in the equation, is made which consumes the greatest amount of reactants, produces the greatest amount of product and produces the greatest amount of heat.
Kit Includes:
3 X 250mL Sodium Hypochlorite 13% Concentrate
1 X 237g Sodium Thiosulfate, anhydrous
DOT Info:
UN1791, Hypochlorite solutions, 8, III, Ltd Qty
Specification
| Brand Name | Innovating Science® |
|---|---|
| Performance Expectations | 5-PS1-3 5-PS1-4 |
| Cross Cutting Concepts | Cause and Effect Scale, Proportion, and Quantity |
| Engineering Practices | Planning and Carrying Out Investigations |
| Box Used | 12663SBOX |
| Width (in) | 6.25 |
| Height (in) | 4 |
| Weight (oz) | 19.9 |
| Weight (lb) | 1.24 |
| Country of Origin | US |
| Harmonization Code | 9023.00.0000 |
| DOT Description | Non regulated |
Related items you may like
Customer Reviews
Be the first to review this product
0%
of customers recommend this product
5 Stars
4 Stars
3 Stars
2 Stars
1 Star
Write Your Own Review
Only registered users can write reviews. Please Sign in or create an account