We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
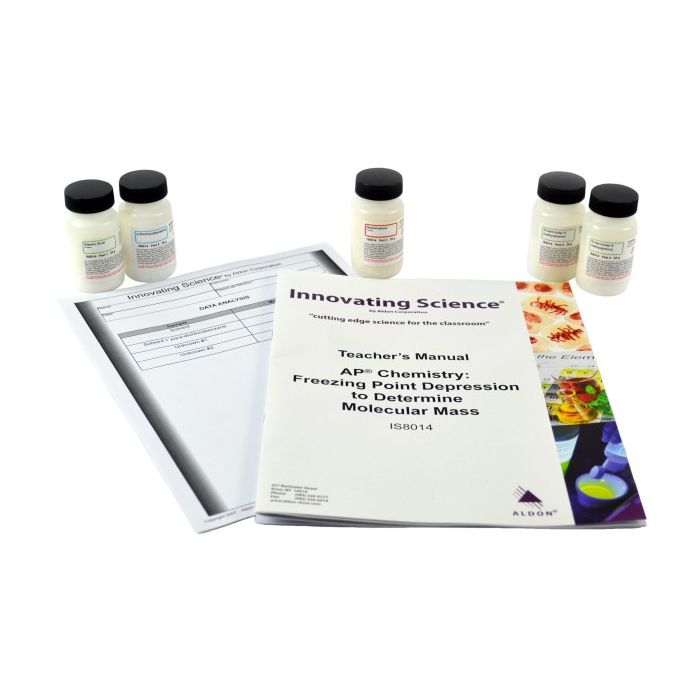
Innovating Science® - Freezing Point Depression AP Chemistry Kit
Rating:
80 % of 100
$46.00
Chemical products can only be purchased by Corporations and Educational Institutions. Private Individuals are not eligible.
Chemical purchases under $50 requires an additional $10 handling fee.
Manufacturer ID - IS8014
Students will learn about acids and bases through a class discussion and then test a dilute acid and dilute base with neutral litmus paper to learn how the paper indicates something is acidic or basic. Once that skill is learned they can move on to test some common items that would be found around their house to determine if they are acids or bases. Optional: Extra litmus paper is included to test more items either from home or around the school building for a greater understanding of acids and bases. The activity contains enough materials for 6 groups of students.
Description
Molecular mass is a parameter, which is useful in determining the identity of an unknown compound. One technique to determine the molecular mass of an unknown is to measure the effect the compound has on the freezing point of a solvent in which the unknown is dissolved. The freezing point of a solution is a colligative property. That is, it is a property which varies based on the number of particles (solute) dissolved in the solvent and not on the chemical makeup of the particles themselves. Other colligative properties, which also can be used to determine molecular mass, are osmotic pressure, vapor pressure and boiling point.
The nonpolar solvent 2,6-di-tert-butyl-4-methylphenol has a freezing point of approximately 70®C. A quantity of para-dichlorobenzene will be dissolved in the solvent and the effect on the freezing point determined. The freezing point depression constant will be calculated for the solvent. The experiment will be repeated with each of two unknowns and the molecular weight of the unknowns will be determined from the freezing point depression.
Kit Includes:
2 x 25g Di-Tert-Butyl-4-Methylphenol Crystals
1 x 25g p-Dichlorobenzene Crystals
1 x 25g Stearic Acid Flakes
1 x 25g Naphthalene Flakes
DOT Info:
Small quantity exemption 173.4
THIS PACKAGE CONFORMS TO 49 CFR 173.4 for domestic highway or rail transport only
 WARNING: This product can expose you to chemicals including p-Dichlorobenzene and Naphthalene, which are known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov.
WARNING: This product can expose you to chemicals including p-Dichlorobenzene and Naphthalene, which are known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov.
The nonpolar solvent 2,6-di-tert-butyl-4-methylphenol has a freezing point of approximately 70®C. A quantity of para-dichlorobenzene will be dissolved in the solvent and the effect on the freezing point determined. The freezing point depression constant will be calculated for the solvent. The experiment will be repeated with each of two unknowns and the molecular weight of the unknowns will be determined from the freezing point depression.
Kit Includes:
2 x 25g Di-Tert-Butyl-4-Methylphenol Crystals
1 x 25g p-Dichlorobenzene Crystals
1 x 25g Stearic Acid Flakes
1 x 25g Naphthalene Flakes
DOT Info:
Small quantity exemption 173.4
THIS PACKAGE CONFORMS TO 49 CFR 173.4 for domestic highway or rail transport only
 WARNING: This product can expose you to chemicals including p-Dichlorobenzene and Naphthalene, which are known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov.
WARNING: This product can expose you to chemicals including p-Dichlorobenzene and Naphthalene, which are known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov.Specification
| Brand Name | Innovating Science® |
|---|---|
| Performance Expectations | 5-PS1-3 5-PS1-4 |
| Cross Cutting Concepts | Cause and Effect Scale, Proportion, and Quantity |
| Engineering Practices | Planning and Carrying Out Investigations |
| Box Used | 12663SBOX |
| Width (in) | 6.25 |
| Height (in) | 4 |
| Weight (oz) | 19.9 |
| Weight (lb) | 1.24 |
| Country of Origin | US |
| Harmonization Code | 9023.00.0000 |
| DOT Description | Non regulated |
Related items you may like
Customer Reviews
Be the first to review this product
0%
of customers recommend this product
5 Stars
4 Stars
3 Stars
2 Stars
1 Star
Write Your Own Review
Only registered users can write reviews. Please Sign in or create an account